

Pfizer, BioNTech and Moderna, which are leading mRNA companies, have launched COVID-19 vaccines one after another, breaking the bottleneck of nucleic acid. Chinese pharmaceutical companies have also received a lot of good news in this field. Many COVID-19 vaccines have received clinical approval, and it is believed that it will not be long before domestic mRNA vaccines are released on the market. As we know, it is extremely important for pharmaceutical enterprises to build a stable supply chain for a drug. Based on the unpredictable international situation, it is particularly important to realize the localization of mRNA industry chain. It is gratifying that the nucleic acid industry chain is being constantly improved, including DNA design, enzymes, lipids, related reagents, equipment consumables related to upstream fermentation and downstream separation and purification, almost all of which can be provided by relevant enterprises. It can be said that nucleic acid drugs in front of the stars of the sea has long predicted its future can be, it will become the most beautiful son of the pharmaceutical industry.
MRNA drug introduction
The basic principle of mRNA drugs is to inject modified mRNA into the body, which can express corresponding protein products in the cytoplasm, so as to exert therapeutic effect. Taking the NOVEL coronavirus mRNA vaccine as an example, the mRNA encoding novel coronavirusS protein is directly injected into the body, which can synthesize S protein in vivo, simulate virus infection and stimulate the body to produce antibodies, thus achieving the prevention of Novel coronavirus infection. In theory, mRNA can express any protein and can be used for protein-based therapies such as preventive vaccines, cancer vaccines, antibodies and protein replacement therapies.
MRNA sequence design and LNP delivery system are the most important steps in mRNA vaccine production. Sequence design can regulate and optimize the expression efficiency of mRNA, which is the basis of nucleic acid vaccine. An effective delivery system is essential for mRNA vaccines to function. The structure of mRNA and LNP delivery system will be introduced emphatically below.
MRNA structure introduction
The main sequence of mature mRNA is the coding region, and there are non-coding regions in its upstream 5 'end and downstream 3' end, and the structural part is the 5 'end cap, 5' end UTR, CDS, 3 'end UTR and 3' end Poly (A) tail.
The cap structure is important for stabilizing mRNA and its translation because it blocks the 5 'end and protects it from exonuclease hydrolysis.
The 5 'Untranslated region (5' UTR) is a short sequence between the hat and the starting codon of the coding region, including the sequence that marks the beginning of translation.
Coding Sequence (ORF region) encoding the primary structure of a protein from the start codon to the end codon cutoff. Each of these three bases forms a codon that codes for an amino acid. The secondary structure of coding region and codon selection may affect translation efficiency. Too many secondary structures and rare codons can slow down translation, so genetically engineered proteins are optimized for host codon preference.
The 3 'untranslated region is the transcription sequence after termination of the codon, which contains the add-on signal. The 3 'untranslated regions are also involved in translation regulation. For example, many micrornas can bind to the 3' untranslated regions of target gene mRNA and down-regulate the expression of target genes by degrading or inhibiting the translation process.
The 3 'ends and the tail are A polymeric A sequence. Mature mRNA generally has poly A tail with 20-200 bases in length at its 3 'end, which can prevent exonuclease degradation and can also be used as A marker of the nuclear pore transport system, which is related to the transport of mature mRNA to cytoplasm through the nuclear pore.
Familiar with the role of each part of mRNA structure, provides a theoretical basis for the optimization of sequence, through the accumulation of experimental data to achieve the purpose of design.
LNP delivery system introduction
How to specifically deliver mRNA into target cells needs to solve three difficulties: extracellular barrier, endosomal escape and intracellular immunity. Only after this triple test can the mRNA vaccine reach the intracellular target site and finally function. LNP is the main technique used in mRNA delivery at present.
Lipid Nanoparticle (LNP) is a kind of Nanoparticle formed from lipids. The structure of LNP is a vesicle composed of phospholipid bilayer. By loading the nucleic acid drug into LNP, it protects the coated nucleic acid drug from degradation and clearance, and facilitates its transmembrane transport to the target site. Its composition is: cationic lipid, neutral auxiliary lipid, cholesterol, polyethylene glycol modified lipid.
In the preparation of LNP, it mainly depends on self-assembly ability, that is, lipid components spontaneously organize into nanostructured entities through molecular interaction. First, the negatively charged nucleic acid and positively charged lipids are bonded by static electricity, and then assembled by hydrophobic and van der Waals interactions between lipid components to form LNP.
The traditional preparation method of LNP is two-phase mixture and then the particle size is controlled by homogenizing technology (high pressure homogenizer, high pressure micro-jet) or extrusion technology (polycarbonate membrane filter). The nanoparticles formed a multilayer structure in which the mRNA molecules were sandwiched in concentric lipid bilayer. The encapsulation rate of this method is generally not high, and it requires high precision and high specialization equipment, which leads to the difficulty of process amplification.
Another method is ethanol dilution. Various lipids were dissolved in ethanol, mRNA was dissolved in acidic water buffer, and the two phases were quickly mixed. By diluting the ethanol phase, the solubility of lipids decreased, and gradually precipitated and solidified in the mixed solution to form lipid nanoparticles. Meanwhile, the mRNA was efficiently encapsulated.
At present, the commercial mass production of mRNA/LNP uses microfluidic or T-junction hybrid technology, which is fast and simple, and has obvious advantages for vaccines and other low-dose drugs.
The Seth nano plasmid assembly system, infusion pump, which provides the core patent technology not only in the resistance under the condition of certain pressure can be precise and smooth conveying required materials, and can meet the requirements of GMP regulations, the unique design of microfluidic mix of nano particles and mRNA, and can be accurate to ensure the particle size and morphology. It is currently playing a key role in the preparation of nanomedicine by a number of biological companies focusing on drug delivery systems.

Unique AutoMCD nano plasmid assembly system
Overall production process of mRNA vaccine
The overall mRNA production process includes DNA plasmid production, mRNA in vitro transcription and purification, mRNA-LNP preparation and preparation filling.
1. Construction of strain: transfer plasmid containing target nucleic acid sequence into EScherichia coli. The nucleic acid sequence here can be extracted from the virus, but the safer and more effective method is to directly connect to the plasmid according to the optimization design of published nucleic acid sequence, avoiding direct contact with the virus;
2. Fermentation: storage and fermentation under appropriate conditions, so that it can replicate hundreds of millions of plasmids;
3. Plasmid purification: After fermentation, enzymes or other chemical substances are added to crack the cell wall of E. coli, and plasmid is collected and purified;
4. Plasmid DNA: Through steps 1 to 3, plasmid DNA is finally harvested.
5. Linearization of plasmid DNA: adding restriction endonucase to cut straight viral genes from circular plasmids and remove residual bacterial and plasmid fragments;
6. In vitro transcription: according to the "central principle", DNA as template transcription into mRNA;
7. Capping and purification: complete the structure completion of mRNA by capping and tail, and remove the incomplete impurity fragments by purification.
8. MRNA stock solution: Through 5 to 6 steps, the final harvest of mRNA stock solution.
9. LNP packaging: Lipid raw materials are prepared and mixed with mRNA stock solution to form lipid nanoparticles through precise control.
10.TFF (tangential flow filtration) : The harvested lipid nanoparticles are concentrated and replaced by tangential flow filtration;
11. Sterilization filtration: the final sterilization filtration of the concentrated lipid nanoparticles;
12. Mrna-lnp: Through steps 9 to 11, mrNA-LNP was finally harvested
Production process, purification as an important part of the work efficiency directly affects the success or failure of the whole process, so the stable and efficient purification system is particularly important, the following for you to recommend the Yingsais purification instrument series of products to make your purification work become easy no worries.
Instrument is introduced
As an instrument manufacturer integrating r&d, production and sales, Suzhou Invensys has developed nucleic acid synthesis and purification instruments comparable to imported equipment, perfect localization. It was quickly recognized by large CDMO and small nucleic acid drug enterprises in China. We not only provide stable and efficient nucleic acid synthesis instrument, purification instrument, HPLC series of instruments and equipment, at the same time, the company invested heavily in building application service platform, to provide customers with a full range of services and overall solutions.
Unique AutoPure purification system
Unique AutoPure purification system is a purification system developed and produced by the company independently. Due to its mature process, the sales volume has increased year after year since its launch, making it the first choice for biopharmaceutical enterprises. In March this year, Unique Autopure Pilot600 was launched. It is a pilot-scale desktop chromatography system, which can provide two configurations in accordance with GMP or non-GMP two different working environments. The system has a wide range of working flow, pressure resistance and detection. It can meet the needs of small scale RESEARCH and development, process amplification and small scale GMP production of different chromatography types.

Unique AutoPure purification system
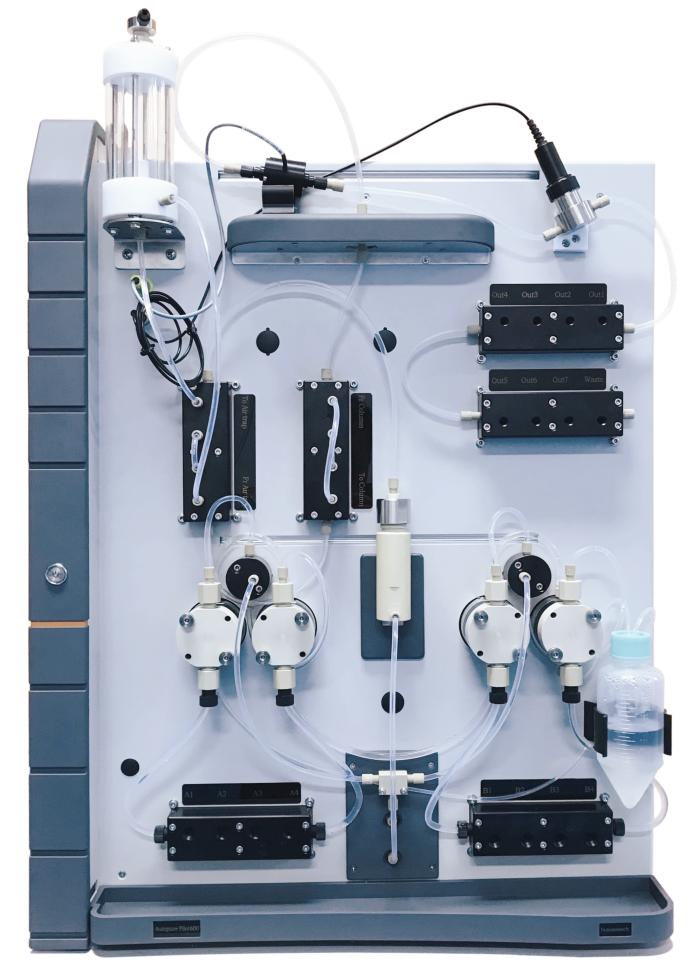
Unique AutoPure Pilot600 system
About Inscinstech
Suzhou Inscinstech Intelligent Technology Co., LTD. (Inscinstech) is an innovative high-tech enterprise focusing on biological separation technology. Founded in 2017, Inscinstech owns numerous patents and computer software Copyrights. The company has established research and development centers in Suzhou, Beijing and Boston, USA, with a sound product research and development system and an international standard technical research and development team. The company's technical team has more than ten years of r&d, engineering and industrialization experience in laboratory sample pre-processing, chromatographic, spectral and mass spectrometry analytical instruments, automation equipment, machine vision technology and other fields, to provide customers with a full range of intelligent solutions from sample separation, pre-processing to laboratory chemical analysis.
Disclaimer:
This article is for reference only and does not constitute investment advice. Investors act accordingly at their own risk and remain neutral in their judgment of the statements and opinions contained herein without giving any warranties, express or implied, as to the accuracy, reliability or completeness of the contents contained herein. The reader is requested for reference only and assumes full responsibility. This public number published all kinds of articles to share, if there is infringement please contact us, we will delete.