

Engaged in medical equipment industry for many years as a move brick, in the past few years, in my web search antibody drug information retrieval occupied the extremely high frequency, like contains PD 1, CAR - T, ADC, double article information always appear often, and at the same time because the outbreak of the new champions league suddenly hits, people found a new direction, This is nucleic acid medicine, which is called the third revolution of biomedicine.
When it comes to nucleic acid drugs, we have to mention the central principle. Central dogma is the process by which genetic material is transcribed from DNA into RNA, which is translated into protein, and this is the law followed by all organisms with cellular structures.
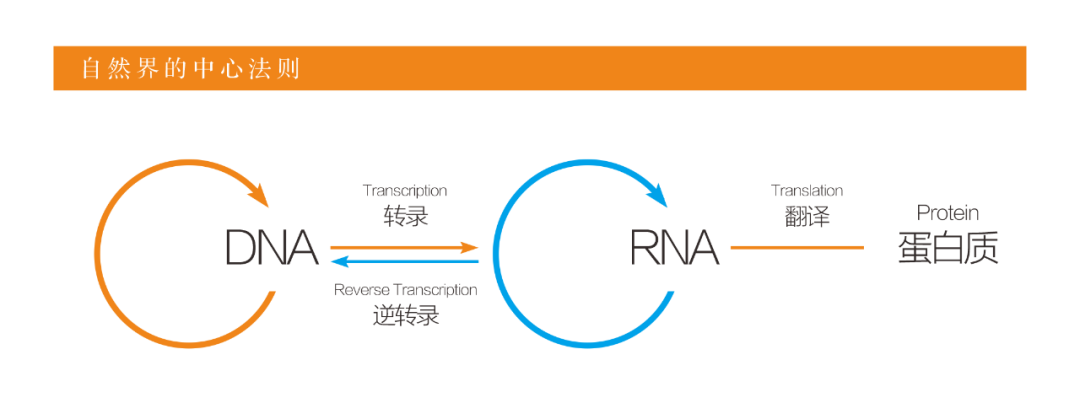
Nucleic acid drugs are different from traditional drugs and antibody drugs, which act directly on DNA or RNA level of drugs. Due to the different sequence length and synthesis methods, it is divided into small nucleic acid drugs (also known as oligonucleotides, obtained by chemical synthesis, generally less than 100nt, usually 20-30nt) and nucleic acid drugs (obtained by in vitro transcription, thousands of nt different). This analysis is of small nucleic acid drugs.
Small nucleic acid drugs mainly include antisense oligonucleotide (ASO), small interfering RNA (siRNA), RNA Aptamer and so on. The discovery of GalNAc and LNPs delivery system has greatly improved organ targeting and cell uptake efficiency of small nucleic acid drugs, and accelerated the rapid development of small nucleic acid drugs. To date, a total of 16 RNA drugs have been approved, including 9 ASO, 4 siRNA, 2 mRNA vaccines and 1 adapter. Six ASO, three siRNA and two mRNA vaccines have been approved since 2016. Novartis's blockbuster new nucleic acid drugs, known as "lipid-lowering magic weapons", have been approved in Europe and the United States. Generally speaking, the global nucleic acid drugs are still mostly in the early stage of research. From 2018 to 2022, there were 88 small nucleic acid drugs trading events, involving a total amount of more than 18 billion US dollars, which shows that the active index in the field of small nucleic acid can be seen.
The production process
Oligonucleotide production process can be divided into two parts: synthesis and purification.
Oligonucleotide synthesis is a process from scratch, in the current general synthetic methods for solid method, its bonding in advance according to the first nucleic acid monomer on the solid carrier, subsequent free monomer and reaction, complete a monomer, the synthesis of such circulation, until after the last monomer reaction, which get a oligonucleotide.
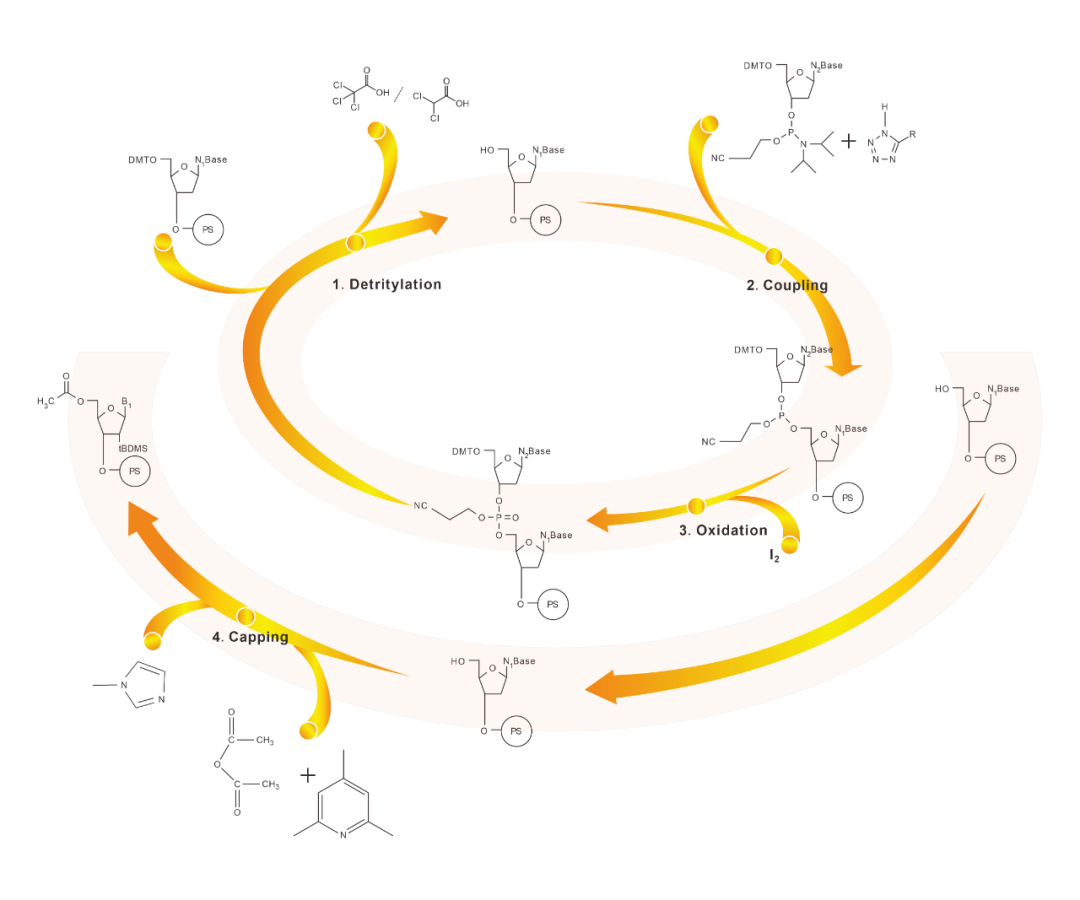
Introduction to synthesis Principle
The synthesis of each monomer is divided into four steps: deprotection, coupling, oxidation and capping.
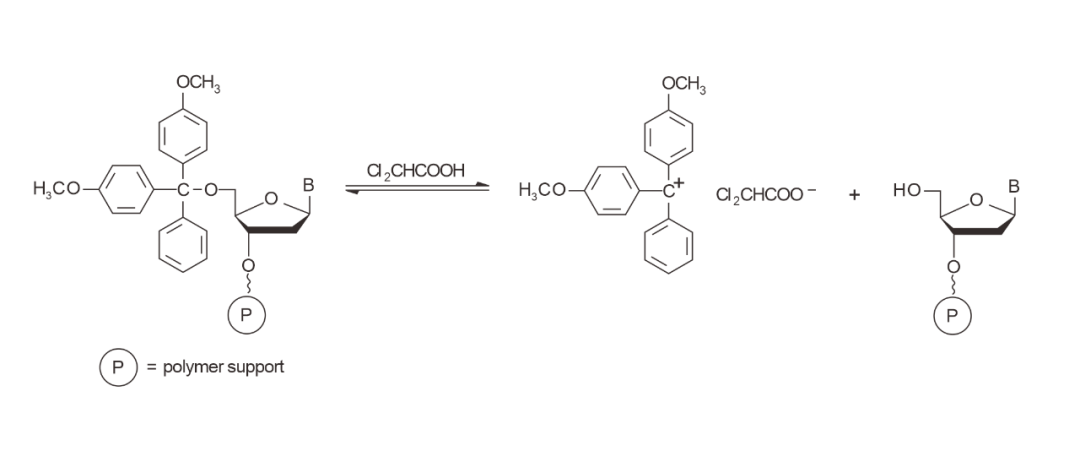
01 Detritylation
Deprotection means that the DMT group is removed from the terminal of the solid carrier and the hydroxyl group is left bare for coupling with the later monomer.
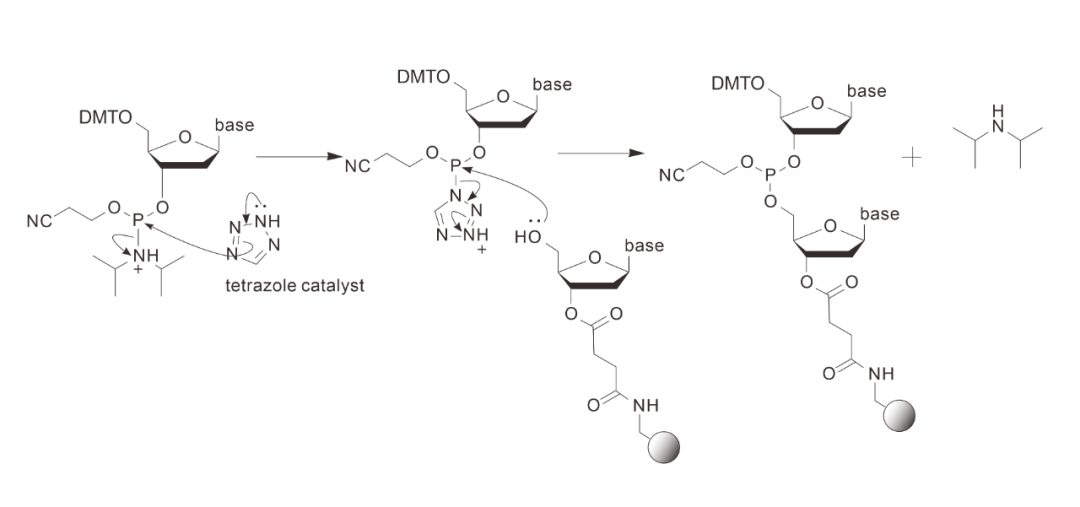
02 Coupling reactions
By coupling, the activator converts the newly added free monomer into a tetrazole derivative, and then forms a 3'-5' phosphite triester with the 5' hydroxyl group exposed on the carrier.
Attention! Complete anhydrous conditions are essential for the efficiency of this step.
03 Oxidation/Thiolation
In pyridine, triphosphite is oxidized to triphosphate with iodine/water. Instead of iodine/water as an oxidant, thiocarpic acid bonds can be introduced at this stage.
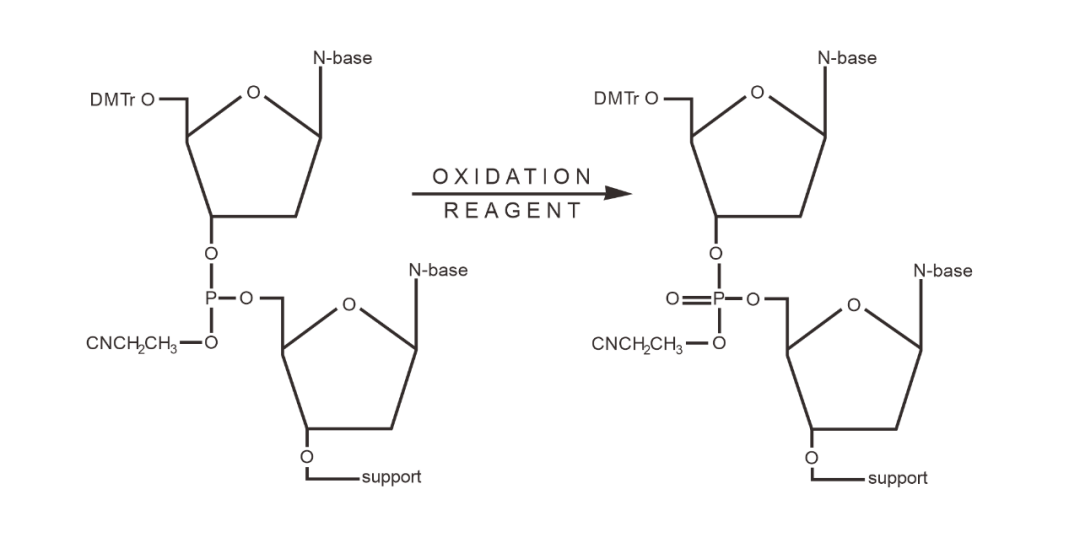
04 Capping
The unreacted 5' -hydroxyl group is sealed to form acylates, preventing the formation of non-target oligonucleotide products.
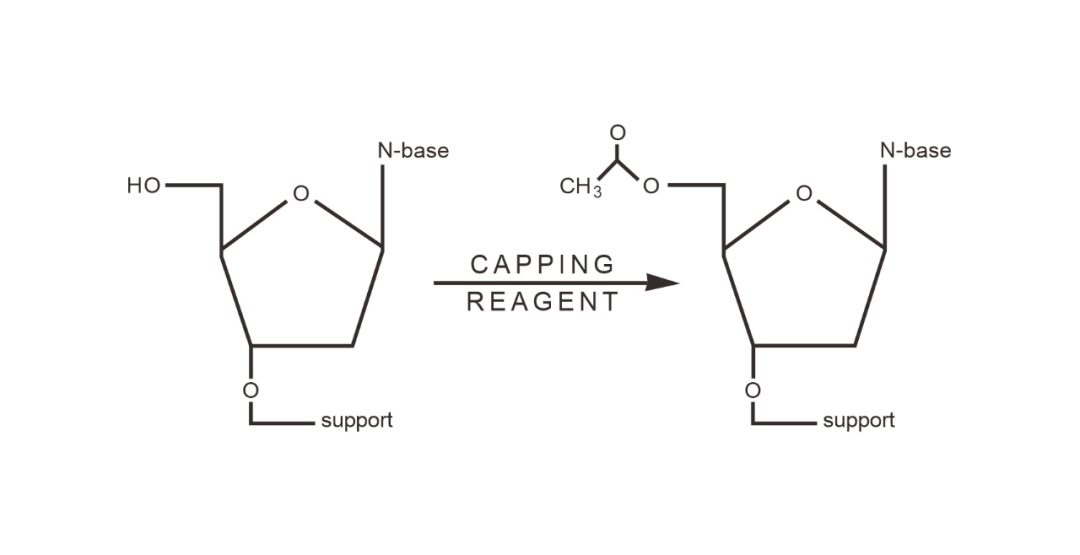
Introduction to purification Principle
Due to the chemical synthesis process, the reaction efficiency and the purity of raw materials, production of synthetic product is inevitable impurities, its main performance for single or multiple oligonucleotide differences, protection groups retain and removal of purine, these groups or sequences of change leads to the impurity molecules with qualitative differences between the target product, main show is:
Phosphate groups: The static charge of an oligonucleotide depends on the number of phosphate or modification groups, the number of bases, and whether the secondary structure shields the charged group;
DMTon/ OFF: DMT is a strongly hydrophobic group that interacts with reversed-phase/hydrophobic chromatography. Therefore, it can be used to separate full-length sequences with DMT.
Thiogeneration: When the oxygen atom in the phosphate group is replaced by the sulfur atom, the polarity of the negatively charged group increases and binds more strongly to the anion exchange filler at most pH conditions;
Methylation: When oxygen atoms are replaced by methyl groups, hydrophobicity increases;
Therefore, purification can use the hydrophobicity or charge difference between the packing and oligonucleotide to change the elution conditions or gradient to separate and collect different components, and finally achieve the purpose of purifying the target product.
Instrument is introduced
As an instrument manufacturer integrating r&d, production and sales, Suzhou Invensys has developed nucleic acid synthesis and purification instruments comparable to imported equipment, perfect localization. It was quickly recognized by large CDMO and small nucleic acid drug enterprises in China. We not only provide stable and efficient nucleic acid synthesis instrument, purification instrument, HPLC series of instruments and equipment, at the same time, the company invested heavily in building CMC platform, aiming to provide customers with a full range of services and overall solutions.
Large-scale nucleic acid synthesizer
The Unique AutoOligo® large-scale nucleic acid synthesizer has been well received by customers since its launch in September last year. The software team of the company holds the principle of "customer first" and takes the user's usage habits into full consideration. The method template and stage module are built into the software. Users can get the required method by simply calling the module and adjusting parameters. In terms of hardware, the input module of the instrument to the power module and the component pipelines involved in the detection module are strictly screened and precisely calculated, providing a guarantee for the optimal synthesis efficiency.

Unique AutoOligo is a flexible, intuitive, automatic oligonucleotide synthesizer for the rapid synthesis of 1μmol-12mmol nucleic acid samples. It is suitable for clinical research, pharmaceutical development and molecular diagnostic probe synthesis.
Unique AutoOligo is available in two models:
Unique AutoOligo25, synthesis of 1~100μmol
Unique AutoOligo100, synthesis of 50μmol~9mmol
Unique AutoOligo150, synthesis of 50μmol~12mmol
Unique AutoOligo meets new synthetic chemistry technologies and ensures a high cost-effectiveness ratio and high-quality synthetic results.
Unique AutoOligo can use up to 14 monomers simultaneously, continuously and automatically switching between one or three monomers
Unique AutoOligo adopts a high-precision metering pump drive system compatible with reagents for nucleic acid synthesis. Unique AutoOligo uses flow-through reactor technology and flow-through solid-phase column synthesis technology to significantly reduce reagent consumption, ensure accurate control of reaction speed and contact time, and improve the synthesis efficiency and facilitate linear amplification.
Unique AutoOliogo System workstation has a powerful data management System, to provide you with a complete nucleic acid synthesis method development platform.
purification system
Unique AutoPure purification system is a purification system developed and produced by the company independently. Due to its mature process, the sales volume has increased year after year since its launch, making it the first choice for biopharmaceutical enterprises. In March this year, Unique Autopure Pilot600 was launched. It is a pilot-scale desktop chromatography system, which can provide two configurations in accordance with GMP or non-GMP two different working environments. The system has a wide range of working flow, pressure resistance and detection. It can meet the needs of small scale RESEARCH and development, process amplification and small scale GMP production of different chromatography types.
System performance parameters
Project | Specifications |
The biggest pressure | 0-27.5MPa(AutoPure25) 0-10MPa(AutoPure100) 0-5MPa(AutoPure150) 0-3.5MPa(AutoPure300) |
Flow range | 0.001 ml/min - 25.00 ml/min(AutoPure25) 0.001 ml/min - 100.00 ml/min(AutoPure100) 0.001 ml/min - 150.00 ml/min(AutoPure150) 0.001 ml/min - 300.00 ml/min(AutoPure300) |
Flow accuracy | ±1.2% @1-100ml/min |
Flow repeatability | 0.5 % RSD |
The wavelength | 280nm/260nm/254nm select a fixed wavelength(Unique L1) 200-600nm range two fixed wavelengths are selected(Unique L2) 200-400nm range choose any two wavelengths(Unique M402) 200-600nm range choose any four wavelengths(Unique M604) 200-800nm range choose any two wavelengths(Unique M804) |
Noise | 1 X10 - 4AU(Unique M402/M604/M804) 2 X10 - 5AU(Unique L1/2) |
Drift | 1 X10 - 3AU/Hr(Unique M402/M604/M804) 5 X10 - 4AU/Hr(Unique L1/2) |
Wavelength indication error | ±2nm(Unique M402/M604/M804) ±1nm(Unique L1/2) |
Wavelength repeatability | 1nm(Unique M402/M604/M804) 0.1nm(Unique L1/2) |
PH | ±0.1 |
Temperature accuracy | ±0.5℃ |
Electrical conductivity | ±0.02mS/cn或2% |
High performance liquid chromatography system
L-1000 series high performance liquid chromatography is widely used in medicine, food, environment and other fields. High precision liquid chromatography pump adopts DMP patented technology, unique pressure compensation method, extremely low pressure pulsation, provides stable and accurate fluid for the system, making the repeatability of chromatographic analysis greatly improved. DAD full-spectrum direct reading UV-Vis detector provides detection or full-spectrum data at multiple wavelengths simultaneously, providing qualitative, quantitative spectral and chromatographic data for analysis.
Case sharing
The following are the result chromatograms of 63MER sequence synthesized by us for customers, including 63MER mass spectrometry, purified chromatograms, crude HPLC chromatograms, and pure HPLC chromatograms.
Application instruments:
· Nucleic acid synthesizer: Inscinstech AutoOligo100
· Nucleic acid purification instrument: Inscinstech AutoPure100
· High Performance liquid chromatography: Inscinstech L-1000 HPLC
· LCMS: ThermoFisher LTQ
Mass spectrometry analysis: the target molecular weight is 19342, the peak molecular weight of mass spectrometry peak is 19344, 19374 peak, which is the ionic impurity peak introduced by the mobile phase separated by LC-MS.
Chromatographic analysis: the purity of crude product was 46.3% and single coupling efficiency was 98.8%. After a simple purification, the purity is 97.803%, to meet the needs of customers for purity.
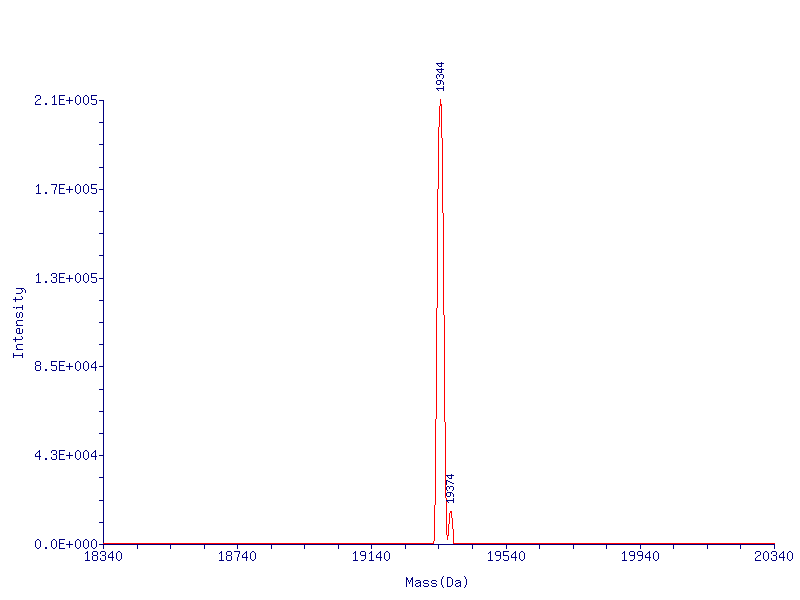
63 killing mass spectrogram
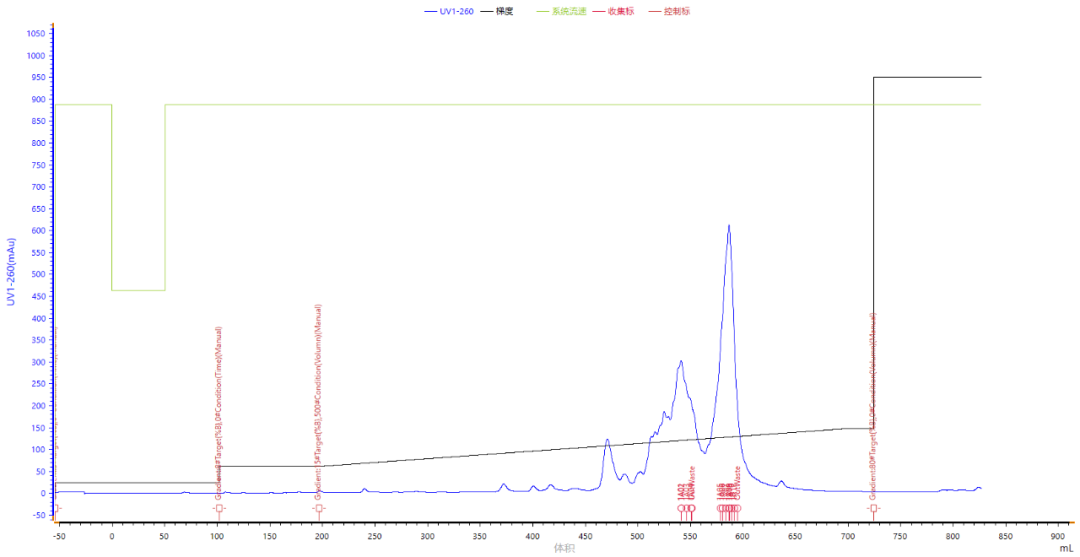
Purification of map
About Inscinstech
Suzhou Inscinstech Intelligent Technology Co., LTD. (Inscinstech) is an innovative high-tech enterprise focusing on biological separation technology. Founded in 2017, Inscinstech owns numerous patents and computer software Copyrights. The company has established research and development centers in Suzhou, Beijing and Boston, USA, with a sound product research and development system and an international standard technical research and development team. The company's technical team has more than ten years of r&d, engineering and industrialization experience in laboratory sample pre-processing, chromatographic, spectral and mass spectrometry analytical instruments, automation equipment, machine vision technology and other fields, to provide customers with a full range of intelligent solutions from sample separation, pre-processing to laboratory chemical analysis.
Disclaimer:
This article is for reference only and does not constitute investment advice. Investors act accordingly at their own risk and remain neutral in their judgment of the statements and opinions contained herein without giving any warranties, express or implied, as to the accuracy, reliability or completeness of the contents contained herein. The reader is requested for reference only and assumes full responsibility. This public number published all kinds of articles to share, if there is infringement please contact us, we will delete.